George Maison
George Maison, Irving Porush and Charles Thiel invented the first pressurized metered dose inhaler (MDI). Developed at Riker Laboratories and introduced in 1956 for the management of asthma, it quickly gained widespread acceptance as the first convenient, portable inhaler that effectively delivered medicine to the lungs. The MDI has saved countless patients’ lives and improved the quality of life for hundreds of millions more.
Maison was born in Frankfort, Kentucky, in 1911. He studied physiology at Northwestern University, where he earned his bachelor’s degree in 1930, his master’s degree in 1934 and his medical doctorate in 1935. After graduating, he taught physiology at several universities.
During World War II, from 1942 through 1945, Maison served in the U.S. Army Air Forces and was chief of a unit in the air technical service command's engineering division. He received the Legion of Merit in 1945 for his work in perfecting the anti-G suit worn by fighter pilots to prevent blackout when pulling out of a dive.
In 1945, Maison began teaching pharmacology at Boston University, and he eventually became the head of the pharmacology department. He established himself there as an innovator, contributing to the development of Veriloid, a drug used to treat hypertension. When he left the university in 1952, he joined Riker Laboratories, a wholly owned subsidiary of Rexall Drug Co., as director of research and development. In 1953, Maison became Riker’s president.
The inspiration behind the invention of the MDI came from a conversation Maison had with his daughter, 13-year-old Susie Maison. Susie had asthma, and she depended on a type of nebulizer that was difficult to handle. Like many nebulizers available at the time, it had a fragile squeeze bulb, which was challenging to operate. While such nebulizers could be relatively efficient at delivering drugs into the lungs’ airways, there was a significant need for a solution that was more user friendly and easier to manage. Susie asked her father if asthma medication could be delivered in a spray, like aerosol cans of hairspray.
Maison immediately began to explore this intriguing idea. He first collaborated with Porush, who was the head chemist at Riker. Porush consulted with Rexall’s cosmetics laboratory, which provided him information about aerosol equipment, propellants and supplies. Soon after, Porush developed an MDI prototype using soda bottles and chlorofluorocarbon refrigerants, and he formulated solutions using epinephrine or isoproterenol, rapidly acting drugs that open the airways and make it easier for patients with asthma to breathe. Thiel, another of the company’s chemists, then created an innovative suspension of the medication in a liquefied gas propellant, increasing the amount of the drug that would be delivered to the lung.
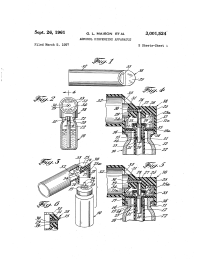
In its finished form, Maison, Porush and Thiel’s device consisted of a reservoir with a mixture of medication and liquefied gas propellant; a metering valve to facilitate delivery of a specific amount of the reservoir’s contents; and a spray actuator to control atomization of the drug. When the valve was pressed, the metered dose of the drug was ejected and flowed into the valve stem. At the spray actuator, it was atomized and released as a fine aerosol plume.
In June 1955, the MDI was tested for safety, and in early 1956, clinical research was published that highlighted its effectiveness, with the majority of patients experiencing excellent symptom relief. The product, described as a rescue inhaler, was approved by the Food and Drug Administration in March 1956, making it the first such device to receive approval for use by asthma patients.
Riker Laboratories marketed the MDI as the Medihaler, which was available in two versions. Medihaler Epi contained epinephrine and Medihaler Iso contained isoproterenol. The small, portable design of the Medihaler revolutionized asthma management, bringing nearly instant relief from respiratory distress in an easy-to-use, highly effective system.
“There were no commercial inhaled therapies considered robust enough to be a mainstay of asthma therapy at the time the metered dose inhaler was invented,” said Anthony Hickey, emeritus professor of molecular pharmaceutics at the University of North Carolina at Chapel Hill Eshelman School of Pharmacy. “There had been many therapies based on unique nebulizers and dry powder inhalers, but most had equivocal dose delivery and would have been considered unsuitable for the delivery of potent pharmacological agents. The MDI could deliver a potent drug in low doses with known pharmacology from a device that accurately and reproducibly metered it to assure safety and efficacy.”
Following his time as president of Riker Laboratories, in 1966, Maison joined Dart Industries, the parent company of Riker Laboratories, where he served as vice president and staff officer. In addition to making a breakthrough in managing asthma, the MDI became essential for treating other lung conditions, including chronic obstructive pulmonary disease. Maison, Porush and Thiel’s invention also later inspired a wealth of innovation through many technical and pharmacological adaptations and advances. In 2014, it was reported that more than 2,000 people were taking doses from an MDI every second. In 2020, in the U.S. alone, an estimated 144 million MDIs were sold, and in 2021, the global MDI industry was valued at $16.3 billion. Its value was expected to increase to $22.1 billion by 2031.